Sample Preparation & Logistics
Please consult our "How to Order Tests" and "Test Information" sections.
General requirements
Biological samples for clinical diagnostic purpose must comply Packing instruction under UN3373, Biological substances Category.
Below instructions are sourced from Packing instruction 650 under IATA Dangerous Goods Regulations so please be well-acquainted with the following.
When the biological samples are packed in accordance with this packing instruction, no other requirements in these Regulations need be met.
The packagings must be of good quality, strong enough to withstand the shocks and loadings normally encountered during transport, including trans-shipment between transport units. Packagings must be constructed and closed so as to prevent any loss of contents that might be caused under normal conditions of transport, by vibration, or by changes in temperature, humidity or pressure.
The packaging must consist of three components:
⒜ a primary receptable(s);
⒝ a secondary packaging; and
⒞ a rigid outer packaging
Primary receptacles must be packed in secondary packagings in such a way that, under normal conditions of transport, they cannot break, be punctured or leak their contents into the secondary packaging. Secondary packagings must be secured in outer packagings with suitable cushioning material. Any leakage of the contents must not compromise the integrity of the cushioning material or of the outer packaging.
Refrigerate or frozen specimens: Cool gel pack or dry ice
- When gel pack or dry ice is used to keep specimens cold, all applicable requirements of these Regulations must be met. When used, gel pack or dry ice must be placed outside the secondary packagings or in the outer packaging. Interior supports must be provided to secure the secondary packagings in the original position after the gel pack or dry ice has dissipated. If dry ice is used, the packaging must be designed and constructed to permit the release of carbon dioxide gas to prevent a build-up of pressure that could rupture the packagings.
- The primary receptacle and the secondary packaging must maintain their integrity at the temperature of the refrigerant used as well as the temperature and pressures, which could result if refrigeration were to be lost.
UN3373, Specimen handling
| Category | Ambient / Refrigerated / Frozen | |
|---|---|---|
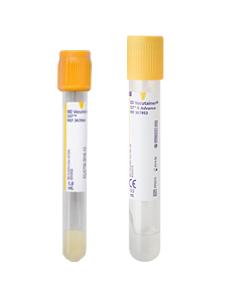
| Tube labelling |
1. After sample collection, there must be more than 2 identifier labelled on the tube 2. Please attach the label clearly on the tube so that it does not fall off. 3. When there are more than 2 sample tubes, label must be attached to each tube. 4. Patient’s information on the labels and requisition form (when required) must be identical. |
 |
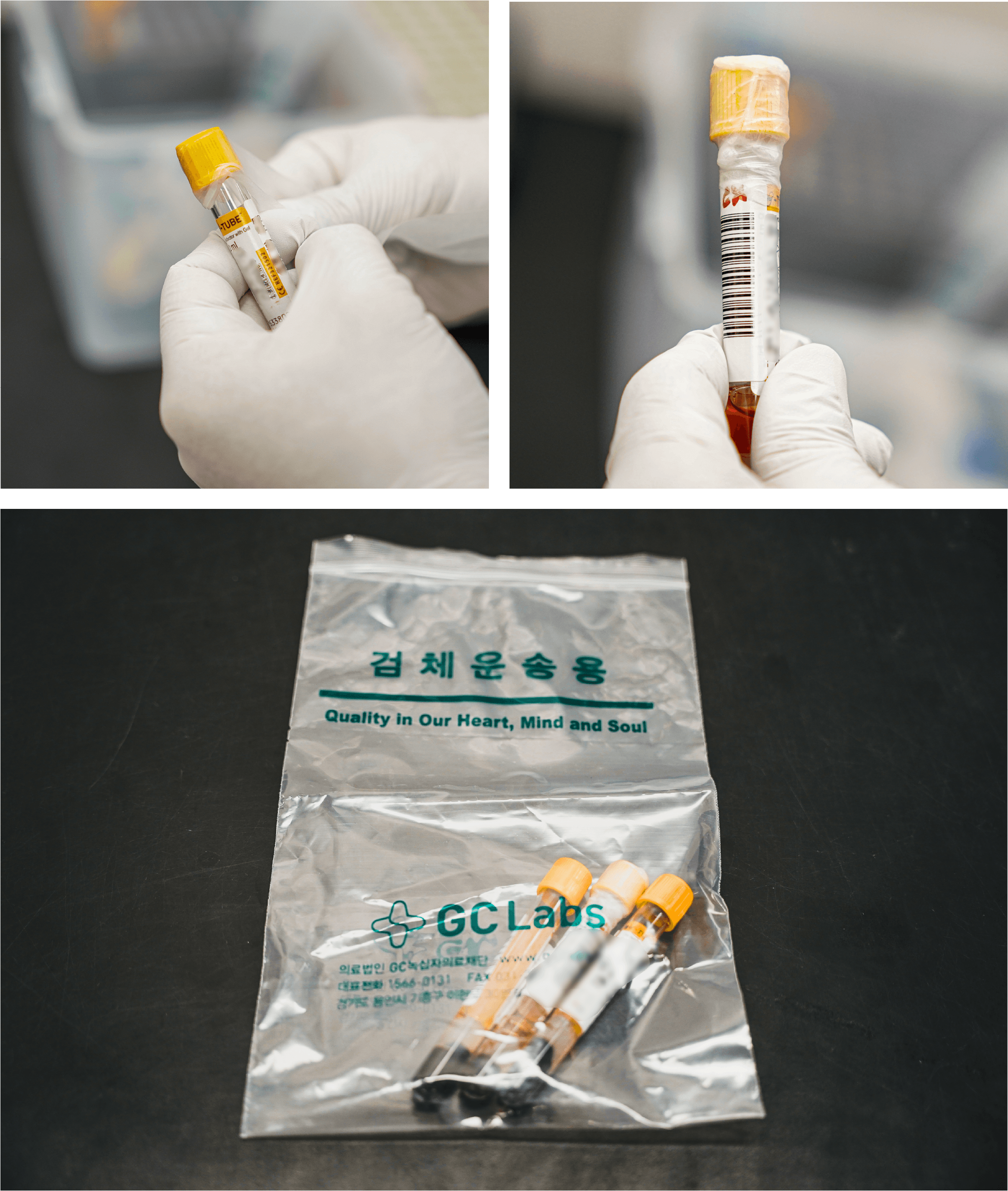
| Tube handling |
After sample is collected, tube must be perfectly sealed. Use Parafilm to wrap around the closed cap to avoid any leakage or contamination. Biohazard bag are paper or plastic bags specifically designed for the safe containment, transportation, and disposal of diagnostic and infectious materials. Sample tubes must be packaged in such a way that they do not come into contact with any external objects. In the case of diagnostic samples, these must be contained in a sterile environment to prevent them from becoming contaminated, and to maintain their viability for testing. |
 |
UN3373, Specimen handling
| Category | Ambient | Refrigerated | Frozen |
|---|---|---|---|
| Packing |
1. Fill the primary receptable (biohazard bag) with samples and seal. 2. Place the primary receptable (biohazard bag) in the secondary packaging and seal. 3. Close and seal the outer packaging. |
1. Fill the primary receptable (biohazard bag) with samples and seal. 2. Place cold Gel Pack at the bottom of the secondary packaging. 3. Place the primary receptable (biohazard bag) on top of the cold Gel Pack. 4. Place cold Gel Packs surrounding the primary receptable (biohazard bag) and seal the secondary packaging. 5. Close and seal the outer packaging. |
1. Fill the primary receptable (biohazard bag) with samples and seal. 2. Place the primary receptable (biohazard bag) in the secondary packaging. 3. Place dry ice surrounding the primary receptable (biohazard bag) and seal the secondary packaging. 4. Close and seal the outer packaging. |
| Marking |
1. Proper shipping name (PSN) :Biological substances Category B 2. Full name and address of Shipper and Consignee |
1. Proper shipping name (PSN) :Biological substances Category B 2. Full name and address of Shipper and Consignee |
1. Proper shipping name (PSN) :Biological substances Category B 2. Full name and address of Shipper and Consignee 3. Net weight of dry ice |
| Labelling |
1. UN 3373 label 2. Package Orientation label (x2) |
1. UN 3373 label 2. Package Orientation label (x2) |
1. UN 3373 label 2. Package Orientation label (x2) 3. UN 1845 label (mention net weight) |
| Marking & Labelling (example) | 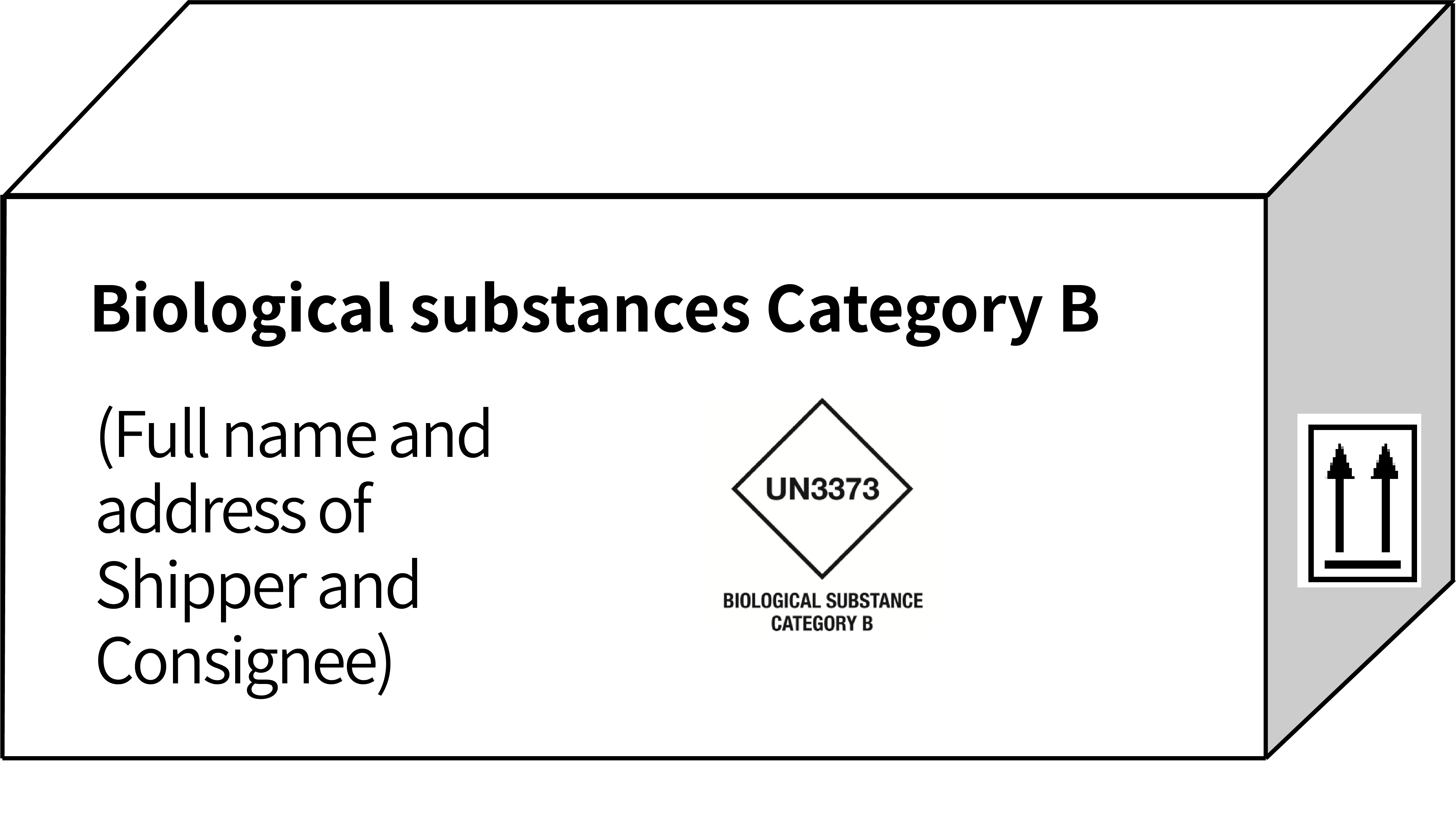 |
 |
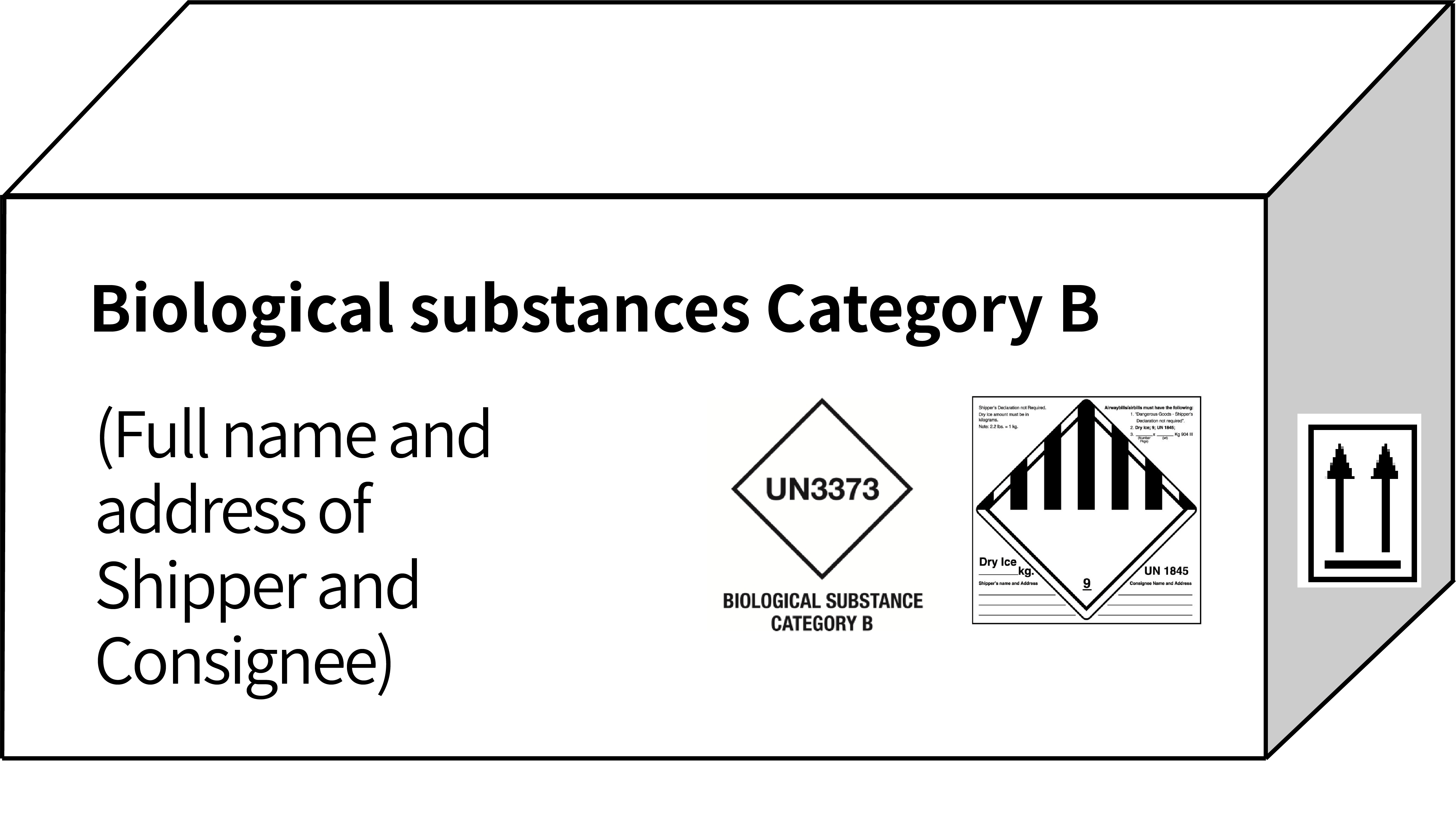 |
| Documentation |
1. Airway Bill 2. Commerical invoice 3. Declaration letter 4. Requisition form (when required) 5. Informed consent form (when required) |
1. Airway Bill 2. Commerical invoice 3. Declaration letter 4. Requisition form (when required) 5. Informed consent form (when required) |
1. Airway Bill 2. Commerical invoice 3. Declaration letter 4. Requisition form (when required) 5. Informed consent form (when required) |
Shipping address
All sample transportation is self-arranged by the shipper. Please prepare all required documents for safe transportation.
- Please scan all the documents and send to gclabsob@gclabs.co.kr. This pre-alert lets GC Labs to track and assist for your shipment.
- Make sure you send the original copies along with the sample package to the following address.
- GC Labs
- Add : 107, Ihyeon-ro 30beon-gil, Giheung-gu, Yongin-si, Gyeonggi-do, Korea(R.O.K)
- Tel : +82-31-260-0607
- Email : gclabsob@gclabs.co.kr

